Catalog
-
Catalog
- Agriculture
- Apparel
- Automobiles & Motorcycles
- Beauty & Personal Care
- Business Services
- Chemicals
- Construction & Real Estate
- Consumer Electronics
- Electrical Equipment & Supplies
- Electronic Components & Supplies
- Energy
- Environment
- Excess Inventory
- Fashion Accessories
- Food & Beverage
- Furniture
- Gifts & Crafts
- Hardware
- Health & Medical
- Home & Garden
- Home Appliances
- Lights & Lighting
- Luggage, Bags & Cases
- Machinery, Hardware & Tools
- Measurement & Analysis Instruments
- Mechanical Parts & Fabrication Services
- Minerals & Metallurgy
- Office & School Supplies
- Packaging & Printing
- Rubber & Plastics
- Security & Protection
- Service Equipment
- Shoes & Accessories
- Sports & Entertainment
- Telecommunications
- Textiles & Leather Products
- Timepieces, Jewelry, Eyewear
- Tools
- Toys & Hobbies
- Transportation
Filters
Search
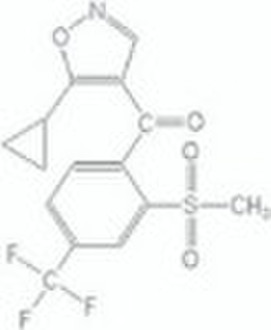
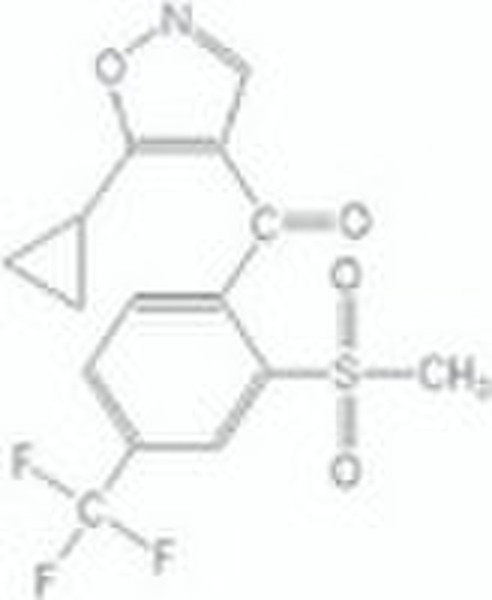
isoxaflutole/agrochemical products

Michael Wang
Contact person
Basic Information
| Classification | Herbicide |
|---|---|
| CAS No. | 141112-29-0 |
| MF | C15H12F3NO4S |
| Place of Origin | Jiangsu China (Mainland) |
| Purity | 95% |
| Application | pesticide |
| State | Granular |
isoxaflutole TECHand other formations Chemical name : 5-cyclopropyl-4-(2-methylsulfonyl-4-Trifluoromethylbenzoyl)isoxazole Composition:Tech. is c. 98% pure.Form:Off-white or pale yellow solid. M.p.: 140C B.p.: V.p.: 1 ×10-3 mPa (25 C) Henry: 1.87 × 10-5 Pa m3 mol-1 (20 C)S.g./density: 1.590 Solubility: In water 6.2 mg/l (pH 5.5, 20 C).Stability: Stable to heat (14 d at 54 C) and to light. DT50 in water 1 d at pH 7. F.p.: Characteristic:Acute oral LD50 for rats >5000 mg/kg.Skin and eye Acute percutaneous LD50: for rabbits >2000 mg/kg. Not a skin irritant; minimal eye irritation (rabbits). Not a skin sensitiser. Inhalation: (4 h) for rats >5.23 mg/l.Birds: Acute oral LD50 (14 d) for quail and mallard duck >2150 mg/kg; 8-d dietary LC50 >5000 ppm.Fish: Non-toxic at limit of water solubility.Bees: LD50 (oral and contact) >100 μg/bee.Daphnia: Non-toxic at limit of water solubility.Algae: EC50 for Selenastrum capricornutum 0.016 mg/l.Worms: Non-toxic at 1000 mg/kg.Other aquatic spp.: EC50 (96h) for Eastern oyster (Crassostrea virginica) 3.4 mg/l, mysid shrimp (Mysidopsis bahia) 18 μg/l. Usage:Biochemistry: p-Hydroxyphenyl pyruvate dioxygenase inhibitor. This enzyme converts p-hydroxyphenyl pyruvate to homogentisate, a key step in plastoquinone biosynthesis. Inhibition leads to indirect inhibition of carotenoid biosynthesis, giving rise to chlorosis of new growth. Mode of action: Systemic by either root or foliar uptake. Uses: Broad-spectrum grass and broad-leaved weed control in maize. Applied at 75-140 g/ha pre-emergence or pre-plant; the spectrum can be enhanced by mixture with other active ingredients. Phytotoxicity: Compatibility: Animals: Following oral administration, isoxaflutole is rapidly excreted. Plants: Plant metabolism study demonstrated that residue levels at harvest are very low, and comprise mainly a non-toxic metabolite. Soil/environment: In laboratory soil studies, degradation proceeds via hydrolysis and microbial degradation, with final mineralisation to CO2. Isoxaflutole and its major metabolites are potentially mobile in soil under simulated high rainfall; however field studies indicate that residues remain in the surface horizons due to the rate of degradation; after 4 months, virtually no residues remain in the soil.
Delivery terms and packaging
Packaging Detail: 25kg drum Delivery Detail: 15days
Port: Shanghai
Payment term
Letter of credit
Telegraphic transfer
-
Payment Methods
We accept: