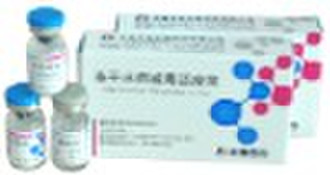
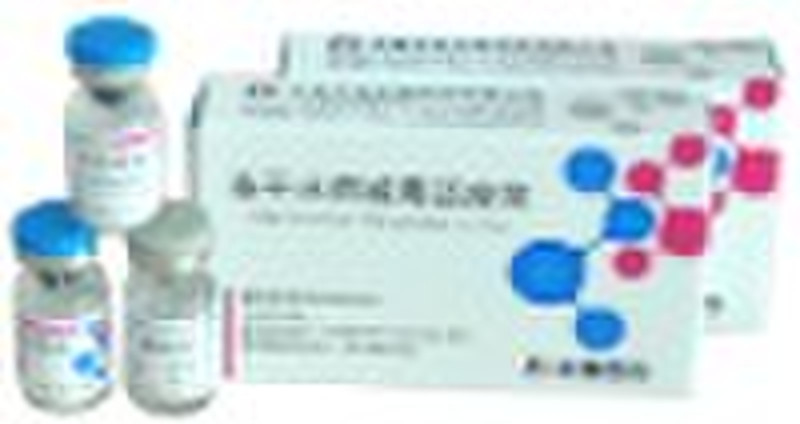
Varicella Vaccine, Live

Joy Zhao
联系人姓名
基本信息
Compositions and dosage form Single dose vial with varicella Oka strain virus not less than 3.3 1g plaque-forming units [PFU] per 0.5 mL dose when reconstituted to a suspension contains manicole 5 mg, dextran 12.5 mg, sucrose 25 mg, trehalose 10 mg, human albumin 5 mg. This whity, fuzzy, lyophilized preparation constituted with a particular stabilizer, containing with live, attenuated varicella-zoster virus (Oka strain) proliferated in human diploid cell (MRC-5), to be reconstituted with sterile diluent gives a semi-hazy to translucent, off-white to pale yellow liquid with no corpus alienum. Indications Varicella Vaccine, Live is a live attenuated virus vaccine indicated in individuals susceptible in varicella and not less than 12 months for prevention from varicella. Function and use Following vaccination, the vaccinee body stimulated can generate the immune response against varicella virus for prevention from varicella. Specifications Reconstituted with 0.5 mL diluent for one vaccinee, a vial of single 0.5mL dose of vaccine contains not less than 3.3 1g PFU of live varicella virus. Administration and dosage Preparation for Administration Withdraw the entire contents of the diluent into a syringe. To avoid excessive foaming, slowly inject all of the diluent in the syringe into the vial of lyophilized vaccine and gently agitate to mix thoroughly. Administered as a single 0.5 mL dose subcutaneously in the deltoid region of the upper arm. Alcohol or other antiseptics may inactivate the attenuated vaccine virus. Administrate just after complete volatilization of the antiseptics away from skin. Recommended dosage Reconstituted with 0.5 ml diluent for one vaccinee, a vial of single 0.5mL dose of vaccine contains not less than 3.3 1g PFU of live varicella virus. Adverse reactions Very low Systematic adverse reactions in all the age groups studied. Adverse reactions on the injection site are usually mild and temporary. In a clinical trial involving 600 infants and children, it was observed that among all vaccines, sporadic papulo-vesicular eruptions appeared in less than 4% incidence and fever (axillary temperature over 37.5 °C) happened in less than 5% cases. This product has no significant difference from the import vaccine. Contraindications Do not administer this vaccine to individuals with a history of anaphylactic reaction to neomycin or any other component of the vaccine. Should not be administered to pregnant females. Should not be administered to individuals treated with steroidal medicine. Do not indicate in individuals suffering from serious diseases (acute or chronic infection), fever and any terminal immune disease. Avoid the use of salicylate within 6 weeks following vaccination of this product. Contraindicated in individuals with known history of congenital immune disease or having closely touched with the family member who has a history of this disease. Do not administer this vaccine to individuals with a total lymphocyte count less than 1200 per mm3 or having other manifestations of cell immunodeficiency. The suspension of this product following reconstitution with the diluent should be used within 30 minutes. Women of child-bearing age should take appropriate conception control at least 3 months following vaccination. Avoid administration with other vaccines within one month following vaccination. Patients with leucocythemia, tumor or immunodeficiency should be restrainedly used under doctors’ guidance. Attenuated live vaccine should be not recommended for use during epidemic seasons. The effects of this product will be cut down for use of whole blood, plasma or immunoglobulin within 5 months before vaccination or within 3 weeks following vaccination. Storage Stored in a refrigerator and transported at 2-8°C against light. Packaging The packing material contacting the pellet of this product directly is 2ml vial made of borosilicate glass. 1vial/person x 1 person/box. One vial per kit accompanied by one vial of sterilized diluent for injection of vaccination. Standard for implementation Chinese Pharmacopoeia” Vol. 3, 2010 and the official standards for registration of this product. Product license number SFDA approval No.GYZZ S20083005 Warnings and precautions For the vaccinee to be kept under medical supervision for 30 minutes following administration with this product. As with any vaccine, adequate treatment provisions, including epinephrine injection (1:1000), should be available for immediate use should an anaphylactic reaction occur. Transmission of vaccinal virus only occurs in extremely rare cases. All vaccinees who might contract with varicella virus, especially who appear skin reactions after vaccination, should avoid contacting with one who is a pregnant woman (especially less than 3 months pregnancy), or a patient with leucocythemia, or a patient being treated with immunosuppressant. Administrate this vaccine subcutaneously. Do not inject intravascularly or intramuscularly. Do not allow to administrate the vaccine under conditions of incomplete reconstitution of this product, vaccine vial cracking and unclear labeling. Avoid alcohol or other antiseptics to contact the vaccine of this product during administration.
交货条款及包装
Packaging Detail: 1vial/person x 1person/box.One vial per kit accompanied by one vial of sterilized diluent for injection of vaccination. Delivery Detail: a month
端口: Beijing
付款条款
Letter of credit
Telegraphic transfer
-
支付方式
我们接受: